-
Drop Foot Rehabilitation System
-
Customer:
-
Awards:
-
Patents:
-
Product approval:
FDA approved
JBD involvement :
Profiling and defining the product, functional ergonometric design, specification of materials including biocompatible ones, locating and purchasing materials, manufacture of feasibility models, prototypes, clinical trial production runs, detailed design and programming CAD files to include all components and sub-assemblies, full engineering design and material specifications, product file and transfer to production.
Additional information:
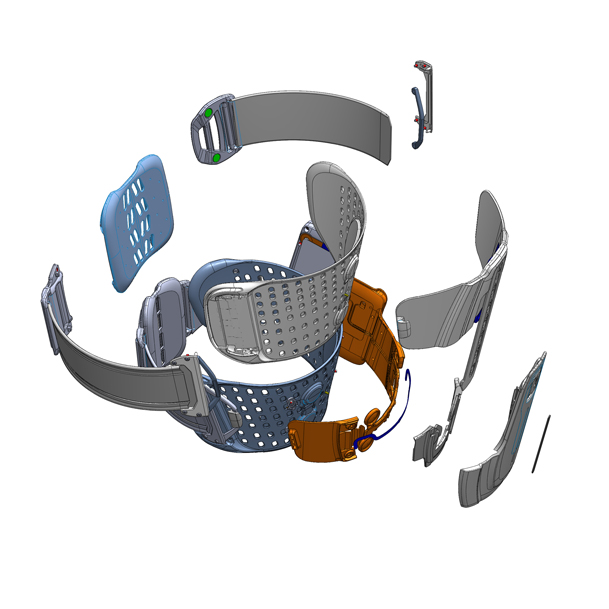
The Bioness L300-Plus system is intended for independent use by people suffering from foot drop and lack of knee control, a condition caused by damage to or disease of the central nervous system. The L300-Plus generates gentle functional electric stimulation (FES) of the muscle and simultaneous activation of the knee and foot, thus facilitating a stable and safe gait.
The system is constructed of plastic, elastomer and textile materials aimed to combine strong structural functionality, springiness, flexibility and a soft touch.
The device is FDA approved.